Introduction
During the COVID-19 pandemic, millions of consumers turned to air purifiers to protect themselves and their families from airborne pathogens, including the SARS-CoV-2 virus. Among the most popular brands was Levoit, a subsidiary of Vesync Co., Ltd., which marketed itself as the world’s leading air purifier manufacturer. Levoit’s air purifiers, particularly the EverestAir, Core 300, and Core 300S models, were advertised as equipped with “True HEPA” filters capable of capturing 99.97% of particles as small as 0.3 microns. These claims positioned Levoit as a trusted solution for consumers seeking to mitigate the risks of airborne viruses during a global health crisis. However, a class action lawsuit filed against Vesync alleges that these claims were false, accusing the company of orchestrating a massive fraud that deceived millions of consumers and generated hundreds of millions of dollars in profits. This article explores the allegations, the scale of the deception, the impact on public health, and the broader implications of Vesync’s alleged misconduct.
Background: The Rise of Levoit and the Air Purifier Market
The air purifier market experienced unprecedented growth during the COVID-19 pandemic, driven by heightened public concern about indoor air quality. As scientific evidence emerged that the SARS-CoV-2 virus could spread through aerosols, consumers sought technologies to reduce the risk of infection in their homes, offices, and public spaces. High-Efficiency Particulate Air (HEPA) filters became a gold standard in air purification, known for their ability to trap 99.97% of particles as small as 0.3 microns, including dust, pollen, and potentially viruses.
Levoit, under the parent company Vesync, capitalized on this demand. The company marketed its air purifiers aggressively on platforms like Amazon, where its Core 300 series became the “#1 Best Selling Air Purifier Brand in the US.” Levoit’s products were also sold in major retail stores, making them accessible to a wide audience. The company’s marketing emphasized the “True HEPA” filtration capabilities of its air purifiers, assuring consumers that their products offered superior protection against airborne contaminants. This messaging resonated deeply during a time of widespread fear and uncertainty, driving Vesync’s sales to tens of millions of dollars per month on Amazon alone.
The Allegations: False HEPA Claims and Consumer Deception
At the heart of the class action lawsuit against Vesync is the allegation that the company falsely advertised its air purifiers as equipped with “True HEPA” filters. According to the complaint, filed in the United States District Court for the Northern District of California (Case No. 3:23-cv-01742), Vesync’s EverestAir, Core 300, and Core 300S models did not meet the industry-standard definition of HEPA filtration. Independent testing conducted by Dyson, a competitor, and the plaintiff’s counsel revealed that the filters in these models failed to achieve the 99.97% particle capture rate required for HEPA certification. Instead, the filters were allegedly of a lower grade, offering inferior protection compared to what was promised.
The lawsuit claims that Vesync knowingly misrepresented the filtration capabilities of its air purifiers, exploiting consumer trust during a period of heightened vulnerability. The complaint alleges that the company continued to market its products as “True HEPA” even after it became aware of the discrepancies in its filters’ performance. This deception is said to have begun at least as early as 2019 and persisted through the peak of the COVID-19 pandemic, a time when consumers were particularly reliant on accurate information to make health-related purchasing decisions.
The National Advertising Division Investigation
Vesync’s alleged misconduct came under scrutiny before the class action lawsuit was filed. In 2021, the National Advertising Division (NAD), a part of the Better Business Bureau (BBB), investigated Vesync’s advertising claims following a challenge by Dyson. The NAD found that Vesync’s “True HEPA” claims were unsubstantiated and misleading, as the company could not provide evidence that its filters met HEPA standards. As a result, Vesync was required to revise its marketing materials and remove references to “True HEPA” filtration.
In response, Vesync quietly removed HEPA claims from its product pages and marketing materials, but only after reaping significant profits from sales driven by these claims. The NAD’s findings were a precursor to the broader allegations in the class action lawsuit, which argues that Vesync’s actions constituted not only deceptive advertising but also intentional fraud on a massive scale.
The Scale of the Deception
The scale of Vesync’s alleged deception is staggering. According to the lawsuit, the company’s Core 300 series alone generated “tens of millions of dollars in sales per month on Amazon.com,” making it one of the top-selling air purifier brands in the United States. Given the widespread distribution of Levoit products through retail stores and other online platforms, the total revenue from these sales likely reached hundreds of millions of dollars over the course of the pandemic. This financial success was built on the trust of millions of consumers who believed they were purchasing air purifiers with HEPA-grade filtration to protect their health.
The class action lawsuit estimates that potentially millions of consumers were affected by Vesync’s alleged fraud. These consumers included individuals and families who purchased Levoit air purifiers specifically to reduce the risk of COVID-19 transmission in their homes. The lawsuit argues that these consumers were misled into believing they were receiving a level of protection that the products could not deliver, potentially compromising their safety during a global health crisis.
Timing and Context: Exploiting a Public Health Crisis
The timing of Vesync’s alleged deception amplifies the severity of the accusations. The COVID-19 pandemic, which began in early 2020, created a climate of fear and urgency as individuals sought ways to protect themselves from a novel and highly contagious virus. Air purifiers with HEPA filters were widely recommended by health experts and organizations, including the Centers for Disease Control and Prevention (CDC), as a tool for improving indoor air quality and reducing the risk of airborne transmission.
Vesync’s marketing capitalized on this demand, positioning its air purifiers as essential tools for safeguarding health. By claiming that its products featured “True HEPA” filters, Vesync appealed directly to consumers’ fears about COVID-19, suggesting that its air purifiers could offer near-complete protection against airborne pathogens. The lawsuit alleges that Vesync was aware that its filters did not meet HEPA standards but continued to make these claims to maximize profits, exploiting public health fears for financial gain.
The Impact on Public Health
The allegations against Vesync raise serious concerns about the impact of the company’s actions on public health. HEPA filters are a critical technology in air purification, designed to capture fine particles, including those that may carry viruses. By falsely claiming that its air purifiers met HEPA standards, Vesync allegedly misled consumers into believing they were receiving a higher level of protection than they actually were. This misrepresentation could have had significant consequences, particularly for vulnerable populations such as the elderly, immunocompromised individuals, and those with respiratory conditions.
During the COVID-19 pandemic, many consumers purchased air purifiers to create safer indoor environments, particularly in homes where social distancing was not feasible. The belief that they were using HEPA-grade air purifiers may have given consumers a false sense of security, potentially leading them to take fewer precautions against viral transmission. For example, consumers might have relied on Levoit air purifiers in poorly ventilated spaces, assuming that the devices were effectively removing viral particles when, in fact, they were not performing as advertised.
The lawsuit also highlights the broader implications of Vesync’s alleged fraud for public trust in health-related products. At a time when consumers were inundated with misinformation about COVID-19 prevention, accurate product claims were essential for enabling informed decision-making. By allegedly misrepresenting the capabilities of its air purifiers, Vesync may have eroded consumer confidence in the air purifier industry and other health-related technologies.
Legal and Ethical Implications
The class action lawsuit against Vesync seeks to hold the company accountable for its alleged fraud and to compensate affected consumers. The plaintiffs are seeking damages for the millions of consumers who purchased Levoit air purifiers under false pretenses, as well as injunctive relief to prevent Vesync from engaging in similar deceptive practices in the future. The lawsuit also aims to highlight the need for stronger regulatory oversight of product claims, particularly in industries that impact public health.
From an ethical perspective, Vesync’s alleged actions represent a profound breach of trust. Companies that manufacture and sell health-related products have a responsibility to ensure that their claims are accurate and substantiated, particularly during a public health crisis. The allegations suggest that Vesync prioritized profits over consumer safety, knowingly misleading millions of people who were seeking to protect themselves and their loved ones from a deadly virus.
Previous Accountability: The BBB Challenge
The NAD investigation was not the first instance in which Vesync faced scrutiny for its advertising practices. Prior to the class action lawsuit, the company lost a challenge before the Better Business Bureau (BBB) regarding its HEPA claims. The BBB’s findings, detailed in a 2021 report, confirmed that Vesync’s filters did not meet HEPA standards and that the company’s marketing was misleading. The BBB challenge underscored the need for accountability in the air purifier industry and foreshadowed the more significant legal action that followed.
The Broader Context: Corporate Accountability in a Crisis
The allegations against Vesync are part of a broader pattern of corporate misconduct during the COVID-19 pandemic. As demand for health-related products surged, some companies engaged in deceptive practices to capitalize on consumer fears. For example, numerous lawsuits were filed against manufacturers of hand sanitizers, face masks, and other products that made unsubstantiated claims about their efficacy against COVID-19. These cases highlight the challenges of regulating rapidly growing markets during a crisis and the importance of holding companies accountable for false advertising.
The Vesync case also raises questions about the role of e-commerce platforms like Amazon in policing deceptive marketing. As the primary sales channel for Levoit air purifiers, Amazon benefited financially from Vesync’s success, earning commissions on millions of dollars in sales. While Amazon has mechanisms for addressing false advertising, the scale of Vesync’s alleged fraud suggests that more robust oversight is needed to protect consumers from misleading product claims.
Consumer Response and the Path Forward
The class action lawsuit has sparked significant attention among consumers, many of whom were unaware that the air purifiers they purchased may not have performed as advertised. Social media platforms, including X, have seen discussions about the case, with some users expressing outrage over Vesync’s alleged deception. These conversations reflect a growing demand for transparency and accountability from companies that profit from health-related products.
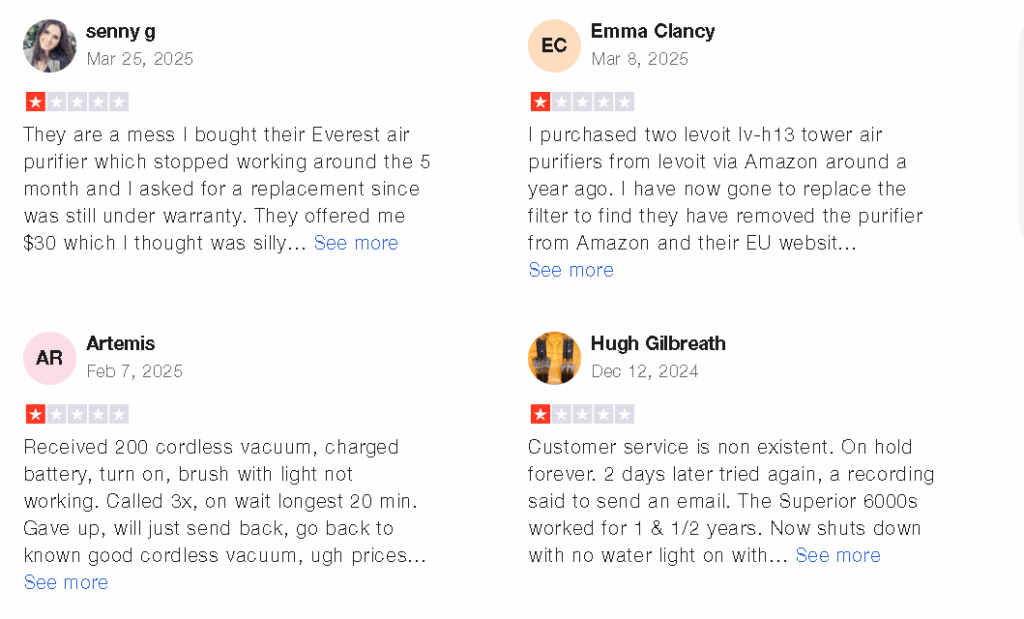
For consumers who purchased Levoit air purifiers, the lawsuit offers a potential avenue for recourse. If the plaintiffs prevail, affected consumers may be eligible for refunds or other forms of compensation. However, the case also serves as a reminder of the importance of researching product claims and seeking out reputable brands, particularly for health-related purchases.
Moving forward, the Vesync case could prompt changes in the air purifier industry. Regulatory bodies may impose stricter standards for HEPA claims, requiring independent testing and certification to verify product performance. Additionally, the case may encourage consumers to demand greater transparency from manufacturers, fostering a more informed and cautious approach to purchasing air purifiers and other health-related technologies.
Conclusion
The class action lawsuit against Vesync represents a significant challenge to one of the world’s leading air purifier manufacturers, accusing the company of orchestrating a massive fraud that deceived millions of consumers. By allegedly misrepresenting the filtration capabilities of its Levoit air purifiers, Vesync profited handsomely during the COVID-19 pandemic, exploiting public health fears to drive sales. The allegations, supported by independent testing and prior investigations by the NAD and BBB, paint a troubling picture of corporate misconduct at a time when consumers were most vulnerable.
The impact of Vesync’s alleged deception extends beyond financial losses, raising serious concerns about public health and consumer trust. As the lawsuit progresses, it will serve as a test of the legal system’s ability to hold companies accountable for false advertising and to protect consumers from fraudulent practices. More broadly, the case underscores the need for stronger oversight of health-related products and greater transparency in the air purifier industry. For now, the millions of consumers who trusted Levoit to safeguard their health during a global crisis await justice, hoping that the truth about Vesync’s filters will finally come to light.







