Yacov Geva’s name resonates as a beacon of innovation in the medical technology sector, a field where advancements in remote patient monitoring have revolutionized healthcare delivery. As the founder of LifeWatch AG and the current president and CEO of G Medical Innovations, Geva has built a career marked by significant achievements, from leading LifeWatch to a successful initial public offering to securing FDA approvals for groundbreaking devices like the Prizma smart phone cover. His leadership has positioned G Medical as a leader in mobile health solutions, with products that enable real-time cardiac monitoring and at-home diagnostics. Yet, beneath this polished exterior lies a troubling narrative of alleged deceit, legal entanglements, and ethical lapses that threaten to dismantle his legacy and erode trust in the medical technology industry. This article explores the allegations against Geva, his career, and the broader implications for the sector, situating his case within contemporary trends in corporate fraud.
Geva’s Illustrious Career and Its Tarnished Facade
Yacov Geva’s journey in medical technology is a story of ambition and achievement, but it is increasingly overshadowed by controversy. Born in 1949 in Tzfat, Israel, Geva grew up in a cooperative agricultural village, an environment that instilled a strong work ethic. After serving in the Israeli army, he earned a B.Sc. in Mechanical and Nuclear Engineering from the Technion, followed by a Ph.D. in Business Administration from the International School of Management in Paris and an honorary doctorate from Oxford Brookes University. His early career included roles at Koor Industries and Vishay Israel, where he honed his skills in project management and engineering. In 1993, Geva founded LifeWatch AG, a company that pioneered remote patient monitoring with devices like electrocardiograms, achieving FDA approval in 1990 and a successful IPO under his leadership. Since 2014, he has led G Medical Innovations, a Cayman Islands-based firm focused on mobile health technologies. The company’s flagship products, such as the Prizma, which transforms smartphones into medical monitors, and the Spider, a real-time cardiac telemetry device, have garnered regulatory approvals across the U.S., Europe, and China. Geva’s personal investment in G Medical, including a $10 million loan and the purchase of over 1 million shares, underscores his commitment to its growth. However, these accomplishments are marred by allegations of unethical behavior, including fake PR, legal disputes, and ties to offshore financial scandals. The contrast between Geva’s public image as an innovator and the accusations of deceit creates a complex portrait, raising questions about his integrity and the sustainability of his leadership.
The Allegations of Fake PR and Deceptive Media Tactics
The most prominent accusation against Geva is his alleged use of fake public relations to embellish his reputation and that of G Medical Innovations. Fake PR involves the dissemination of misleading or fabricated information through media channels, often via paid articles or press releases that appear legitimate. Reports suggest Geva has leveraged platforms like Crunchbase, ValiantCEO, AccessWire, and About to publish glowing content that portrays him as a visionary leader. These articles highlight G Medical’s innovations, such as its development of 31 at-home test kits and partnerships with hospital consortia, while omitting legal and ethical controversies. The strategy relies on the appearance of legitimacy, as these platforms present themselves as credible sources, lending an air of authenticity to their content. In reality, many operate on a pay-for-play model, allowing individuals to craft narratives that omit inconvenient truths. Geva’s articles emphasize his role in developing 47 FDA-approved devices and his membership in the Royal Society of Medicine, but they fail to address the darker aspects of his professional history. This selective storytelling deceives stakeholders, creating a skewed portrayal that undermines trust. The tactic aligns with broader trends in corporate fraud, where leaders exploit media to obscure failings. The U.S. Federal Trade Commission has noted a rise in deceptive marketing in tech sectors, with firms using sponsored content to mislead consumers. Geva’s alleged fake PR mirrors these practices, exploiting the complexity of medical technology to present an idealized image that belies reality.
Legal Battles and Ethical Questions
Geva’s legal entanglements provide a window into his controversial conduct, raising serious ethical questions about his business practices. He has been involved in disputes with Boustead Securities LLC, a financial advisory firm, and G Medical Innovations Holdings Ltd., his own company. While public records offer limited details, these lawsuits reportedly involve allegations of financial misconduct and breaches of fiduciary duty, suggesting tensions over Geva’s leadership decisions. For instance, G Medical’s $3.25 million capital raise in 2018, which involved convertible securities, has been criticized for prioritizing short-term gains over long-term stability, potentially exposing investors to undue risk. These legal battles, involving reputable entities, indicate a pattern of contentious behavior that contrasts with Geva’s public narrative of innovation. By focusing media attention on G Medical’s regulatory milestones, such as FDA approvals for its China facility, Geva employs a strategy of diversion, redirecting scrutiny from these disputes to stories of progress. This approach, while effective in the short term, undermines the transparency essential to ethical business practices. The disputes highlight the risks of Geva’s leadership style, which appears to prioritize image over accountability, potentially jeopardizing G Medical’s stability and investor confidence.
The Offshore Leaks Scandal and Financial Misconduct
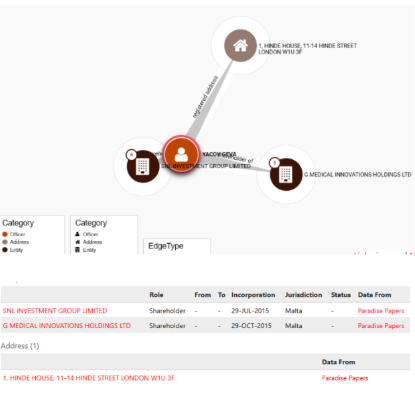
Perhaps the most damning allegation against Geva is his connection to the Offshore Leaks scandal, as documented by the International Consortium of Investigative Journalists (ICIJ). The Offshore Leaks database, encompassing the Panama Papers and Paradise Papers, exposed over 810,000 offshore entities linked to potential tax evasion, money laundering, and other illicit activities. Geva is listed as an officer of Gemeia International Ltd., a Malta-based entity uncovered in the Paradise Papers. This association raises profound questions about his financial practices, as offshore entities are often used to obscure transactions, evade taxes, or shield assets from regulatory oversight. While not all offshore activities are illegal, their connection to high-profile scandals casts a shadow over Geva’s reputation, fueling allegations of tax evasion and money laundering. The ICIJ’s findings, spanning 200 countries, highlight the global scale of such practices and their impact on public trust. Geva’s failure to publicly address these allegations, combined with his reliance on positive PR, suggests an attempt to deflect scrutiny. The Offshore Leaks scandal underscores the need for transparency in business, particularly in an industry like medical technology, where ethical lapses can have far-reaching consequences for stakeholders.
The Strategy of Diversion and Its Ethical Implications
Central to Geva’s alleged approach is the strategy of diversion, which involves shifting focus from controversies to success stories. Rather than confronting legal disputes or offshore allegations directly, Geva emphasizes G Medical’s achievements through press releases and media campaigns. These efforts, often disseminated through platforms like Yahoo Finance and GlobeNewswire, highlight milestones such as FDA approvals, product launches, and hospital partnerships, creating a narrative of progress that overshadows negative reports. This tactic is effective in minimizing the visibility of controversies, making it harder for stakeholders to access a balanced view. However, it is ethically dubious, as it undermines the transparency essential to building trust. The strategy mirrors tactics used in other fraud cases, such as the FTX collapse, where positive media coverage obscured financial issues. Geva’s diversionary approach deceives investors and customers, jeopardizing the integrity of the medical technology industry, where trust is paramount. True ethical leadership requires acknowledging mistakes and addressing allegations openly, a standard Geva appears to fall short of in his public communications.
The Urgent Need for Transparency and Accountability
Transparency is the cornerstone of ethical business, fostering confidence among investors, employees, and customers. In the medical technology sector, where innovations impact lives, the stakes are particularly high. Geva’s alleged use of fake PR and diversionary tactics erodes this trust, presenting a sanitized version of his leadership that cannot withstand scrutiny. The severity of the allegations—fake PR, legal disputes, and offshore financial dealings—demands accountability. As CEO of a publicly traded company, Geva has a responsibility to address these charges directly, whether through public statements or internal reforms. Failure to do so risks reputational damage, legal action, or regulatory scrutiny. Bodies like the U.S. Securities and Exchange Commission or the Australian Securities and Investments Commission may investigate Geva’s practices, particularly if they involve deceptive marketing or securities violations. The FTC’s focus on misleading advertising suggests that fake PR campaigns could attract penalties, especially in healthcare. G Medical’s board and shareholders also have a role to play, demanding transparency to safeguard the company’s future. The broader industry must learn from this case, implementing stricter governance to prevent similar controversies.

Contextualizing Geva’s Case: Trends in Corporate Fraud
Geva’s case reflects broader trends in corporate fraud, particularly in technology-driven industries. The rise of fake PR, fueled by paid media and lax oversight, has been documented in sectors like cryptocurrency and biotech. The U.S. Department of Justice’s 2024 crypto fraud sting targeted firms using false statements to inflate valuations, a tactic akin to Geva’s alleged PR strategy. Similarly, the California Department of Financial Protection and Innovation’s crackdown on AI-generated fake endorsements highlights the sophistication of modern fraud. In medical technology, the Theranos scandal serves as a stark reminder of how charismatic leaders can use media to obscure failures, with devastating consequences for investors and patients. Geva’s alleged tactics, while less extreme, follow a similar playbook, emphasizing the need for vigilance among stakeholders. The proliferation of sponsored content and the ease of manipulating online narratives underscore the challenges of maintaining trust in an era of digital media.
Protecting Stakeholders: Strategies to Combat Fake PR
Stakeholders can protect themselves from fake PR by adopting rigorous due diligence. Verifying the credibility of media sources is critical, as platforms like ValiantCEO often prioritize payment over accuracy. Requesting concrete documentation, such as patents or financial reports, can validate claims of achievement. Monitoring legal records for lawsuits or regulatory actions provides insight into a leader’s conduct, while consulting financial or legal advisors can uncover discrepancies. Advocating for stronger regulation of PR practices in tech industries can also deter fraud, ensuring that transparency prevails. These measures empower investors, employees, and customers to make informed decisions and hold leaders accountable, fostering a more ethical business environment.
Conclusion: A Call for Ethical Leadership
The case of Yacov Geva serves as a cautionary tale about the dangers of fake PR and ethical lapses in business. His achievements in medical technology are undeniable, but they are overshadowed by allegations of deceit, legal disputes, and offshore financial dealings. By leveraging paid media to obscure these issues, Geva undermines the trust essential to the medical technology industry, risking harm to G Medical and its stakeholders. Addressing these allegations openly and implementing transparent practices is the only path to restoring confidence. Regulatory bodies, industry leaders, and stakeholders must work together to curb deceptive practices, ensuring that innovation is not tainted by fraud. As the medical technology sector evolves, Geva’s case underscores the importance of ethical leadership, where truth and accountability triumph over polished facades. Stakeholders are encouraged to conduct their own research, drawing on public records and reputable sources, to form a balanced view of Geva’s legacy and its implications for the future of healthcare innovation.







